Abstract
Background: CTCL represents a rare group of non-Hodgkin lymphomas with substantial negative impact on patient (pt) quality of life and mortality in advanced-stage disease. Mycosis fungoides (MF), the most common subtype of CTCL, and the rarer leukemic variant Sézary syndrome (SS) are distinct subtypes of CTCL. Mogamulizumab is a first-in-class, defucosylated monoclonal antibody directed against C-C chemokine receptor 4 (CCR4), which is highly expressed on malignant T-cells in CTCL. Primary results from the MAVORIC study (data cut-off December 2016), a phase 3 trial comparing mogamulizumab to FDA-approved vorinostat in adults with relapsed/refractory MF/SS, showed mogamulizumab significantly prolonged median progression-free survival compared with vorinostat (7.7 vs 3.1 months, P<0.0001), with a confirmed overall global response rate (comprised of all disease compartments) of 28% and an expected and generally manageable safety profile. This follow-up analysis assessed the safety and efficacy of mogamulizumab based on treatment exposure in order to characterize pts with MF/SS who were able to achieve long-term clinical benefit with mogamulizumab.
Methods: This was an open-label, randomized, international, phase 3 study (NCT01728805). Pts with MF/SS who were treated with ≥1 prior systemic therapy were randomized 1:1 to receive mogamulizumab (1.0 mg/kg, administered once weekly for the first 28-day cycle, then on Days 1 and 15 of subsequent cycles) or oral vorinostat (400 mg daily). In this follow-up analysis (data cut-off September 2017), exposure quartiles were determined, and baseline demographics, confirmed global response rate, and safety were analyzed by exposure group. To detect linear trends across exposure quartiles, frequencies were analyzed using Chi-square test and continuous data were assessed by analysis of variance.
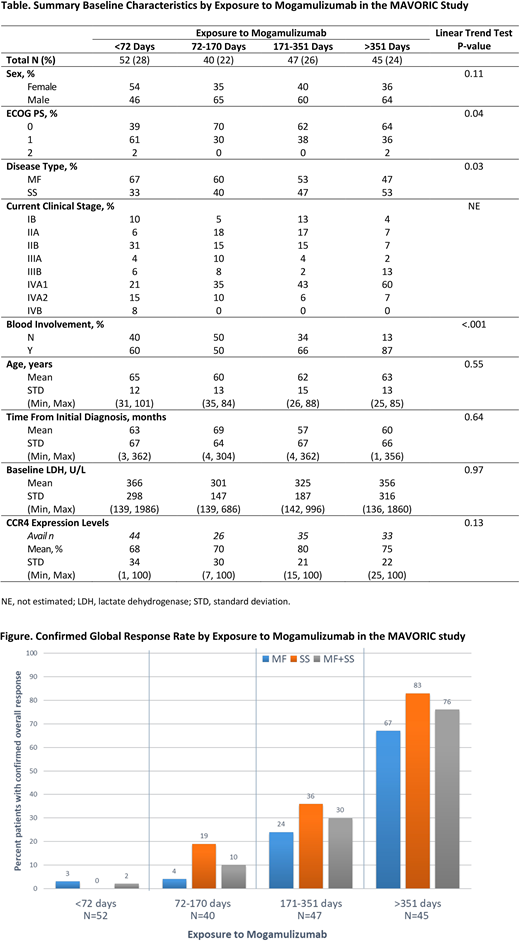
Results: A total of 184 pts randomized to mogamulizumab were included in this analysis. Mean time of mogamulizumab exposure was 275 days (d; standard deviation [SD]: 292 d; range: 1-1617). Based on quartile assessment, >351 d was defined as cut-off for long-term exposure. Baseline characteristics across exposure groups are shown in the Table. Significant trends were observed for baseline Eastern Cooperative Oncology Group performance status (ECOG PS; P=0.04), disease type (P=0.03), and blood involvement (defined as ≥B1 per Olsen et al J Clin Oncol 2011; P<0.001), with long-term pts more likely to have an ECOG PS grade of 0, SS, and/or blood involvement. Confirmed global response rates increased with increasing exposure to mogamulizumab in pts with MF and SS (Figure; trend P<0.001 for MF, SS, or MF+SS). Of all randomized pts exposed to mogamulizumab for >351 d, 76% had a confirmed global response (ie, either complete or partial response). The percentage of pts reporting a treatment-emergent adverse event (AE) or serious AE did not vary with increasing exposure to mogamulizumab (<72 d exposure: 26% of pts with TEAEs and 6% with SAEs; 72-170 d: 18% and 3%, respectively; 171-350 d: 23% and 6%, respectively; >351 d: 21% and 4%, respectively). The most common treatment-related AEs reported by pts after >351 d of exposure to mogamulizumab were drug eruption (9/45 [20%]), thrombocytopenia (5/45 [11%]), stomatitis (4/45 [9%]), and anemia (4/45 [9%]).
Conclusions: This follow-up analysis of the phase 3 MAVORIC study demonstrated mogamulizumab treatment of pts with MF/SS for approximately 1 year was not associated with an increased safety risk. Significant long-term clinical benefit was observed in pts with blood involvement at baseline, regardless of CCR4 expression status. A higher proportion of pts who had long-term (>351 days) exposure attained confirmed global response versus those who had less exposure.
Bagot:Takeda: Membership on an entity's Board of Directors or advisory committees; Innate Pharma: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees; Actelion: Membership on an entity's Board of Directors or advisory committees. Dalle:Kyowa Hakko Kirin Pharmaceutical: Research Funding. Sokol:Mallinckrodt Pharmaceuticals: Consultancy; Seattle Genetics: Consultancy; Spectrum Pharmaceuticals: Consultancy. Tsianakas:Kyowa Kirin: Research Funding. Musiek:Seattle Genetics: Honoraria; Kyowa Kirin: Honoraria; Actelion: Other: Scientific Advisory Committee . Ortiz-Romero:Innate Pharma: Consultancy; Takeda: Consultancy; MEDA: Research Funding; Actelion: Consultancy; 4SC: Consultancy. Poligone:Johnson and Johnson: Research Funding; Kyowa Hakko Kirin: Research Funding; Soligenix: Research Funding; Mallinckrodt: Speakers Bureau; Stemline Therapeutics: Honoraria; Seattle Genetics: Honoraria. Duvic:Clinical Care Options: Consultancy; Soligenix, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Mallinckrddt Pharmaceuticals (formerly Therakos): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Huron Consulting Group: Consultancy; Taiwan Liposome Company LTD: Consultancy; Rhizen Pharma: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Hakko Kirin, Co: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Oncology, LLC: Membership on an entity's Board of Directors or advisory committees; Eisai: Research Funding; UT MD Anderson Cancer Center: Employment; Dr. Reddy's Laboratories (A.K.A. Promius Pharma): Consultancy; Defined Health: Consultancy; Medivir AB: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: Speaker/Preceptor; Guidepoint Global: Consultancy; Jonathan Wood & Associates: Other: Speaker; Celgene Corp: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Evidera, Inc.: Consultancy; Kiniksa Pharmaceuticals: Consultancy; MEDACorp: Consultancy; The Lynx Group: Consultancy; Spatz Foundation: Research Funding; Forty Seven, Inc.: Membership on an entity's Board of Directors or advisory committees; Shape: Research Funding; Aclaris Therapeutics Int'l Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cell Medica Inc.: Consultancy, Honoraria; Allos: Research Funding; American Council on Extracorporeal Photopheresis (ACE): Membership on an entity's Board of Directors or advisory committees; Concert Pharmaceuticals, Inc.: Consultancy; Millennium Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MiRagen Therapeutics: Consultancy; Huya Bioscience Int'l: Consultancy; Array Biopharma: Consultancy, Honoraria; Oncoceuticals: Research Funding; Tetralogics: Research Funding. Elmets:NCI: Research Funding; Veterans Administration: Research Funding; California Wine Grape Association: Research Funding; Soligenix: Research Funding; Elorac: Research Funding; Leo Pharma: Other: Data and Safety Monitoring Board. Leoni:Kyowa Kirin: Employment. Dwyer:Kyowa Kirin: Employment. Sun:Kyowa Kirin: Employment. Nikonova:Kyowa Kirin: Employment. Kim:miRagen: Research Funding; Forty Seven Inc: Research Funding; Kyowa-Kirin-Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innate Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Horizon Pharma: Consultancy, Research Funding; Merck: Research Funding; Soligenix: Research Funding; Eisai: Membership on an entity's Board of Directors or advisory committees, Research Funding; Medivir: Membership on an entity's Board of Directors or advisory committees; Neumedicine: Consultancy, Research Funding; Portola: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Galderma: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Tetralogic: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal